Ethanol
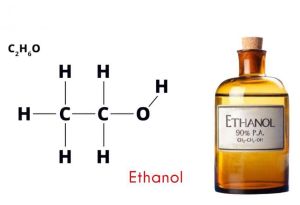
Ethanol, also known as ethyl alcohol or grain alcohol, is a widely used chemical with numerous applications in various industries. Here’s a comprehensive look at ethanol:
Chemical Properties[edit | edit source]
- Chemical Formula: C₂H₅OH
- Molecular Weight: 46.07 g/mol
- Physical State: Colorless, volatile liquid at room temperature
- Boiling Point: 78.37°C (173.1°F)
- Freezing Point: -114.1°C (-173.4°F)
- Solubility: Completely miscible with water and many organic solvents
Production[edit | edit source]
- Fermentation: The most common method of producing ethanol is through the fermentation of sugars by yeast. This process typically involves the following steps:
- Preparation: Sugars are extracted from raw materials such as corn, sugarcane, or other biomass.
- Fermentation: Yeast is added to the sugar solution, converting it into ethanol and carbon dioxide through anaerobic respiration.
- Distillation: The ethanol is then distilled to increase its purity.
- Synthetic Production: Ethanol can also be produced synthetically through the hydration of ethylene in the presence of a catalyst.
Uses and Applications[edit | edit source]
- Fuel: Ethanol is commonly used as a biofuel, either as pure ethanol (E100) or blended with gasoline (e.g., E10, E85). It’s considered a renewable energy source and helps reduce greenhouse gas emissions.
- Beverages: Ethanol is the primary intoxicating ingredient in alcoholic beverages such as beer, wine, and spirits.
- Industrial Solvent: Due to its solvent properties, ethanol is used in the manufacture of pharmaceuticals, cosmetics, and personal care products. It’s also used in the production of paints, coatings, and cleaning agents.
- Antiseptic: Ethanol has antimicrobial properties and is used in hand sanitizers, medical wipes, and disinfectants.
- Chemical Feedstock: Ethanol serves as a starting material for the synthesis of various chemicals, including ethyl acetate, acetic acid, and butadiene.
Safety and Health Effects[edit | edit source]
- Short-term Effects: Moderate consumption of ethanol can cause relaxation and euphoria, but higher doses can lead to intoxication, impaired judgment, and motor skills.
- Long-term Effects: Chronic ethanol consumption can result in liver disease (e.g., cirrhosis), cardiovascular problems, addiction (alcoholism), and various cancers.
- Toxicity: Ethanol poisoning can occur with excessive consumption, leading to symptoms such as vomiting, confusion, respiratory depression, and potentially death.
- Safety Precautions: Proper handling and storage of ethanol are essential to prevent accidental ingestion, inhalation, or skin contact. Use appropriate protective equipment and ensure good ventilation in work areas.
Environmental Impact[edit | edit source]
- Biodegradability: Ethanol is readily biodegradable and generally considered environmentally friendly. However, its production, especially from agricultural sources, can have environmental impacts such as land use changes and water consumption.
- Pollution: Incomplete combustion of ethanol as a fuel can produce pollutants such as acetaldehyde and formaldehyde.
Future Trends and Innovations[edit | edit source]
- Sustainable Production: Research is focused on developing more sustainable methods of ethanol production, including using non-food biomass and waste materials.
- Advanced Biofuels: Ethanol is being explored as a precursor for advanced biofuels and biochemicals, which can further reduce reliance on fossil fuels and lower carbon emissions.
- Innovative Uses: New applications of ethanol in fields like energy storage and hydrogen production are under investigation.
Ethanol is a versatile and important chemical with widespread use in various sectors. While it offers many benefits, responsible consumption and sustainable production practices are crucial to minimizing its potential health and environmental impacts.